| |
 |
 |
| |
| Home | Project | People | Research | Education | Archive | |
 |
Chemogenomics |
[English | Japanese ] |
 |

|
  |
 |
Yoshiji TakemotoProfessor,
Graduate
School of Pharmaceutical Sciences,
Kyoto University |
Creation of Biologically Functional Molecules that Specifically Interact with Proteases
With an aim of establishing the knowledge information infrastructure for genome science by means of organic synthetic chemistry, we are struggling with creation of artificial multi-functional molecules, which work like proteases, and development of catalytic asymmetric reactions and synthetic methods for synthetic compound libraries. Through these studies, novel drug temples as well as unknown pharmacophores that can interact multiply with biologically important macromolecules will be revealed.
1. Design and synthesis of artificial enzyme-like molecules
Taking into account the reaction mechanism of serine-proteases, we designed and synthesized various types of small molecules, which may possess enzymatic functions. As a result, the thiourea derivatives bearing a tertiary amino group were proved to have an excellent catalytic activity as a general acid as well as a general base like enzymes. In addition, we have succeeded in total asymmetric synthesis of (R)-baclofen and a NK-1 receptor antagonist (CP-99,994) using these thiourea catalysts.
2. Development of synthetic method for chiral heterocyclic drug templates
To develop effective inhibitors against HIV- and SARS-proteases, we designed two types of heterocyclic compounds (type 1 and 2) as a drug template, to which a hydroxy group, a guanidine, and an aromotic ring could be introduced at a different position, respectively. The preparation of these drug templates could be achieved stereoselectively by the Ir-catalyzed allylic substitution reaction, which we had already developed.
3. Total synthesis of natural products possessing inhibitory activity against proteasome
The proteasome has become an interesting target for the development of drugs against cancer and Alzheimer's disease. We are now struggling to develop an efficient synthetic method for the total synthesis of omuralide and TMC-95A. During these studies, we have already discovered two key reactions, In-mediated radical cyclization and Rh-catalyzed hydroamidation, for the key intermediates, (3E)-3-alkylidene-γ-lactames, of the targeted molecules. 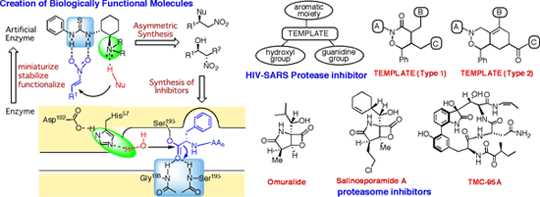
|
Top
| 2007 | 2006 | 2005 | |
|
|

