| |
 |
 |
| |
| Home | Project | People | Research | Education | Archive | |
 |
Environmental Genomics |
[English | Japanese ] |
 |

 |
![Chemical Genomics]](../../img/btn_e_chemo_01.gif)  |
 |
Hiroaki Kato
Professor,
Graduate School of Pharmaceutical Sciences,
Kyoto University |
Structural Biology for Biological Clock
Structural biology
is the study of the architecture and shape of biological
macromolecules and what causes them to have the structures
they have. This subject is of great interest to biologists,
because macromolecules carry out most of the biological
functions, and because typically they are folding into
a specific three-dimensional shape to perform their functions.
We are aiming to understand the function of important proteins
that will be the key to the drug development by structural
biology and to develop the research techniques of structural
biology, especially the crystallization methods for membrane
proteins and the ultra-high-resolution X-ray crystallographic
techniques beyond 1 Å resolution using the synchrotron radiation of SPring-8. We believe that the underlying principle of the function is structure. We are not interested in the structure itself, but in the function. Thus, we are proceeding functional studies by various techniques from molecular biology to physical chemistry as well as protein crystallography.
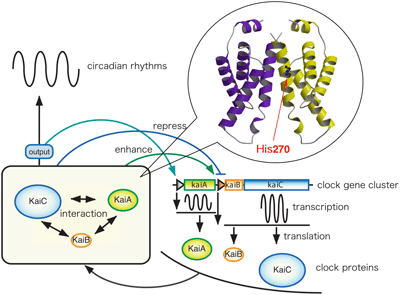
The circadian clock exists in most organisms, and controls the daily activity cycles. In cyanobacteria, that appear on the earth as the first photosynthetic organisms, kaiABC is the clock gene cluster. It is known that the cluster consists of two operons, kaiA, whose product enhances kaiBC promoter activity, and kaiBC, which is repressed by the KaiC protein. We found that the C-terminal domain of KaiA is responsible for its dimer formation, binding to KaiC, enhancing KaiC phosphorylation and generating the circadian oscillations. To understand the circadian clock machinery at the atomic level, we solved the three-dimensional structure of the C-terminal clock oscillator domain of KaiA by X-ray crystallography. We also characterized in KaiA, a functionally essential residue, His270, and examined the roles of several aminoacid residues that have been reported previously to affect circadian rhythm.
Uzumaki et al., Nat. Struc. Mol. Biol., 11, 623-631 (2004)
|
Top
| 2007 | 2006 | 2005 | |
|
|

